Abstract
Background: Core Binding Factor (CBF) leukemia, characterized by inversion (16) or t(8;21) cytogenetic abnormalities, is associated with a relatively favorable prognosis and highly responsive to treatment with fludarabine, cytarabine, and granulocyte colony stimulating factor combination (FLAG) along with either gemtuzumab ozogamicin (FLAG-GO) (Borthakur, Am J Hematol, 2014) or idarubicin (FLAG-IDA) (Borthakur ASH 2013). Response monitoring is facilitated by the use of real time polymerase chain reaction (qPCR) assessment for fusion gene transcripts. In this study, we assess factors underlying disease response kinetics and their predictive utility (including the role of quantitative fusion gene transcript ratio)in determining survival outcomes in CBF AML.
Methods: One hundred and four patients (pts) with CBF AML were treated with frontline sequential regimens of FLAG-GO or FLAG-IDA at MDACC from 2007 to 2014. Baseline and periodic qPCR values were collected for analysis; logarithmic transformations were applied on qPCR data and depth (quality) of response expressed as quantitative changes in log reductions/increases (log (qPCR)). In order to determine the rate of response, random regression was applied on repeated measures of the log (qPCR) for all 104 pts. Regression coefficient for the each individual's PCR response over time was extracted from the model and measured as the slope [rate of log-reduction over time] (or rate of response). Stepwise cox regression models were applied to evaluate factors predicting for survival in the multivariate analysis. Maximally selected log rank statistics (MSLRS) methods were used to determine optimum cut-offs for continuous covariates, wherever applicable.
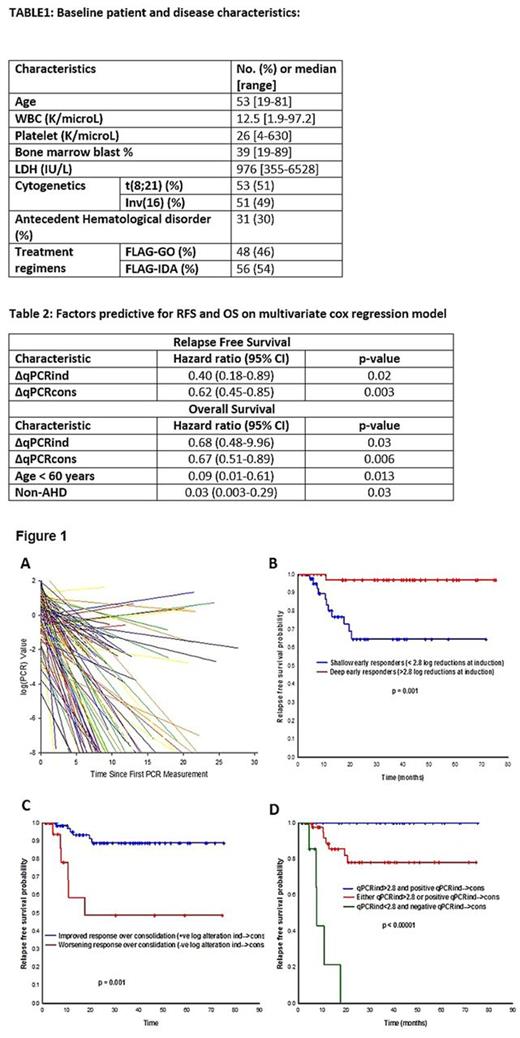
Results: Baseline pt and disease characteristics are outlined in table 1. Median log (qPCR) reduction (∆qPCRind) over first FLAG induction cycle was 2.8 (0.0-7.0). Median number of FLAG consolidation cycles received were 6 (range, 1-6). Median log alteration from post-induction to post-consolidation phase (log (qPCR) end-induction - log (qPCR) end-consolidation = ∆qPCRcons) was 1.4 (-2.9-7.0). Rates of response (slopes) for the duration of treatment course for all cases are plotted in figure 1A. ∆qPCRind correlated strongly with the overall regression slope (p = 0.0012) indicating that depth of initial response predicts for an overall steep slope (i.e., faster rate of response). Also, there was a trend toward significance by regimen type (p = 0.07) and cytogenetics (p = 0.05), with FLAG-IDA and t(8;21) associated with slower rates of response. At a median follow up of 22 (1-76) months among survivors, 92 (88%) pts are alive; 18 (17%) have relapsed (5 pts during consolidation). Depth of initial response did not, however correlate with rate of log (qPCR) response during the consolidation phase of treatment. Among patients in continued remission at the end of consolidation phase, and not treated with further maintenance, RFS did not differ by the presence or absence of a detectable qPCR transcript-at-end-consolidation (p = 0.87). Among analyzed factors, ∆qPCRind, ∆qPCRcons predicted for a statistically significant improved relapse free survival (RFS); ∆qPCRind, ∆qPCRcons, age, and antecedent hematological disorder were significantly associated with overall survival (OS) (table 2). Importantly, rate of response did not predict either for RFS or OS. Patients with a ∆qPCRind of > 2.8 (by MSLRS on RFS) (n = 47, 45%) and a positive ∆qPCRcons (i.e., ∆qPCRcons > 0) (n = 84, 81%) had a significantly improved RFS compared to those without (Figures 1B & 1C, respectively). When accounting for both variables, pts who failed to achieve either of the two qPCR depth-of-response measures had the lowest RFS compared to those achieved at least one; pts who achieved both had the best RFS (Figure 1D).
Conclusions: ∆qPCRind, ∆qPCRcons (both, measures of depth of response) are important predictors of RFS and OS, and quantitative cut-offs derived from our study serve as clinically useful estimates to identify pts at an increased risk of relapse. Patients with inv(16) and those treated with FLAG-GO have a faster rate of response (as measured by qPCR) to treatment. However, neither regimen, cytogenetics, nor rate of response to treatment impact RFS or OS.
Cortes: Sun Pharma: Research Funding; Teva: Research Funding; ARIAD: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Kantarjian: Bristol-Meyers Squibb: Research Funding; ARIAD: Research Funding; Amgen: Research Funding; Novartis: Research Funding; Delta-Fly Pharma: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.